Worldwide
Login- Research
- 1x1 Platforms
- 1x1 Overview
- tDCS Overview
- 1x1 tDCS Device
- 1x1 tES Device
- 1x1 Clinical Trials Device
- 1x1 Accessories
- High Definition Platforms
- HD Overview
- HD-tDCS Overview
- 4x1 HD Adaptor
- MxN-5 REMOTE
- MxN-PRO
- MxN-GO EEG
- HD-Accessories
- REMOTE
- REMOTE Overview
- mini-CT
- SNAP Headgear
- ElectraRx
- MOBILE
- Software
- Neurotargeting Overview
- HD-Explore
- HD-Targets
- HD-Targets-IFS
- tDCS-Explore
- Modeling Service
- Transcranial Focused Ultrasound Stimulation (tFUS)
- Transcranial Pulse Stimulation (TPS)
- Temporal Interference Stimulation (TI)
- Temporal Interference Stimulation (TI)
- Releasenotes
- HIGH DEFINITION ECT
- TRANSCRANIAL PHOTOBIOMODULATION
- transcutaneous spinal DCS (tSDCS)
- Multimodal Solutions
- Multimodal Overview
- tES + PET
- tES + fMRI
- tES + fNIRS
- tES + EEG
- Galvanic Vestibular
- GVS Devices
- GVS Accessories
- Transcranial Magnetic Stimulation (TMS)
- SPRY TMS
- SPRY POINT Navigation
- MEGA-TMS
- Neuro-MEP Software
- TMS compatible EEG
- MEGA-EMG
- MagXite
- Vital Sign Monitoring
- HealthDot
- Mobile EEG
- MOBILE EEG Overview
- SMARTING PRO Line
- SMARTING mobi
- Functional Near Infrared Spectroscopy
- NIRSIT System
- NIRSIT LITE
- NIRSIT ON
- Artinis Systems
- Soterix Medical Workshop
- Soterix Medical Workshop NYC 2026
- Webinars
- Unique tDCS by Soterix Medical
- Home-tDCS Depression Trial
- Soterix Medical Virtual Booth
- Transcutaneous Auricular Vagus Nerve Stimulation (taVNS)
- Pre-Clinical
- Animal DCS Overview
- Animal DCS / tES
- Animal Accessories
- Animal Accessories
- Animal Transcranial Magnetic Stimulation System
- Animal Transcranial Focused Ultrasound Stimulation (tFUS) system
- Linear Current Isolator
- EDUCATION TECHNOLOGY
- Smart Mobility
- Support
- Contact us
- Distributors
- Cart
- Manuals
- Legacy
- Legacy Devices
- Videos
- Configure Products
- Request Product Information
- Device registration
- News Room
- Press
- Events
- Newsletter
- Clinical trials
- Publications
- Webinars
- Images
- About us
- EASYpad™
- EASYstrap
- Elastic Fastener Set
- SNAPpad™
- SNAPstrap
- Carbon Rubber Electrode
- EASYkit
- EASYcase
- Clinical Neuromodulation CART
- Breakout Box
- Banana to Din Adapter
- SMI Signal Isolator
- SNAPheadset
- HD-Electrode
- HD1 Electrode Holder
- HD1-Biosemi Electrode Holder
- HD-Cap
- HDM Electrode Holder
- HDM-Biosemi Electrode Holder
- HD-GEL
- 4x1 Input Cable
- MxN-33 Output Cable
- HD-tES Soft Base MRI Electrode Holder
- HD-tES MRI Electrode Holders
- MxN-33 Cable Disconnector
- SMI Chronos Adapter
- GVS HEADstrap
- GVS Headset
Research High Definition Platforms
High Definition Platforms
 High Definition Platforms
High Definition Platforms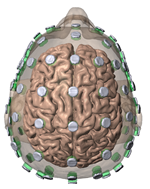

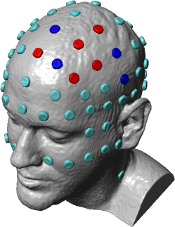
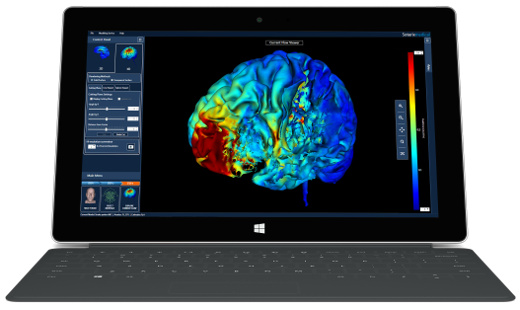
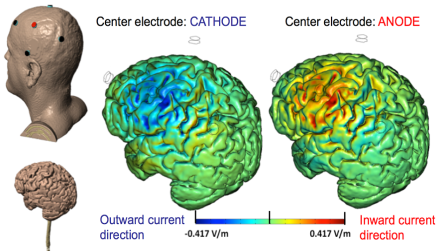 High-Definition transcranial Direct Current Stimulation (HD-tDCS) was invented at The City University of New York with the introduction of the 4x1 HD-tDCS montage (Datta 2009). The 4x1 HD-tDCS montage categorically transformed the possibilities with non-invasive neuromodulation by allowing precise targeting of cortical structures. How focal is 4x1 HD-tDCS? The region of current flow is circumscribed by the area of the 4x ring, such that decreasing ring radius increases focality. Soterix Medical Neurotargeting software makes HD montage design fast and easy, but the innovation did not stop there. Scientists went on to demonstrate that 4x1 HD-tDCS allows for unifocal stimulation, meaning the polarity of the center 1x electrode will determine the direction of neuromodulation under the ring (Villamar 2013). This is in contrast to conventional tDCS where the need for one anode and one cathode always produces bidirectional modulation (even when an extra-cephalic electrode is used). 4x1 HD-tDCS thus provides the ability not only to select a cortical brain region to target, but to modulate the excitability of that brain region with a designed polarity without having to consider return counter-electrode flow.
High-Definition transcranial Direct Current Stimulation (HD-tDCS) was invented at The City University of New York with the introduction of the 4x1 HD-tDCS montage (Datta 2009). The 4x1 HD-tDCS montage categorically transformed the possibilities with non-invasive neuromodulation by allowing precise targeting of cortical structures. How focal is 4x1 HD-tDCS? The region of current flow is circumscribed by the area of the 4x ring, such that decreasing ring radius increases focality. Soterix Medical Neurotargeting software makes HD montage design fast and easy, but the innovation did not stop there. Scientists went on to demonstrate that 4x1 HD-tDCS allows for unifocal stimulation, meaning the polarity of the center 1x electrode will determine the direction of neuromodulation under the ring (Villamar 2013). This is in contrast to conventional tDCS where the need for one anode and one cathode always produces bidirectional modulation (even when an extra-cephalic electrode is used). 4x1 HD-tDCS thus provides the ability not only to select a cortical brain region to target, but to modulate the excitability of that brain region with a designed polarity without having to consider return counter-electrode flow.