
MEGA-TMS™
Transcranial Magnetic Stimulators
The most advanced TMS with unmatched strength and control.
Soterix Medical® was founded to support the science of non-invasive electrical stimulation. Building on deep expertise in stimulation technologies, Soterix Medical has since expanded its offerings to TMS and tFUS, and synergistic monitoring technologies. From exclusive High-Definition systems, Neurotargeting planning software, high power TMS, to a fully integrated tFUS solution, researchers choose Soterix Medical® when stimulation quality cannot be compromised.
Soterix Medical® is committed to providing patients and physicians with the most advanced, user-friendly, and effective neuromodulation treatments. Our clinical tDCS platforms are the only systems incorporating the latest, most robust technology. Unique electrode and head-gear snap-in technology make consistent and comfortable stimulation “a snap” for patients and caregivers. Our TENS solutions incorporate the widest pulse width for deeper pain relief. Our clinical TMS solution is the most ergonomic and portable in its class.

Soterix Medical Inc., the global leader in non-invasive stimulation and integrated brain monitoring technologies, announced today clearance from the U.S. Food & Drug Administration (FDA) for the SPRY Transcranial Magnetic Stimulation (TMS) Therapy. SPRY TMS is indicated for the treatment of Major Depressive Disorder in adult patients, who have failed to achieve satisfactory improvement from prior antidepressant medication in the current episode.

Soterix Medical Inc. the global leader in stimulation and synergistic monitoring technologies, announced today it has received a 510(k) clearance from the U.S. Food & Drug Administration for its Intraoperative Neurophysiologic Monitoring (IOM) system, MEGA-IOM. The system provides unmatched integrated control of central and peripheral nervous systems to reduce postoperative risk and improve surgical outcomes.

Soterix Medical Inc. the global leader in non-invasive brain stimulation and synergistic monitoring technologies, announced the launch of MxN-GO EEG, a combined High Definition transcranial Electrical Stimulation (HD-tES) and EEG system with a unique untethered design. The system is intended for research applications requiring electrical stimulation and recording of brain activity in mobile and natural environments.

Soterix Medical Inc. announces the launch of a Phase-2 clinical trial that will evaluate the combination of REMOTE-tDCS and a brain training program for the treatment of long COVID. The trial will be administered through NYU Langone Health's home-based remotely supervised (RS) tDCS program, which includes a clinical service that is available to patients across the United States.

Soterix Medical Inc. the global leader in non-invasive stimulation and synergistic brain imaging technologies, announces a new clinical trial of home-based auricular Vagus Nerve Stimulation (taVNS) for individuals who experience post-COVID neuropsychiatric symptoms, like fatigue, headache, or anxiety.

Soterix Medical Inc. the global leader in non-invasive stimulation and synergistic brain imaging technologies, is pleased to report expanded clinical trials of its proprietary neuromodulation technologies for Alzheimer's disease and Mild Cognitive Impairment.

Soterix Medical Inc. announces it has received FDA Investigational Device Exception (IDE) to launch a trial of transcranial Direct Current Stimulation-Limited Total Energy (tDCS-LTE) neuromodulation at-home for patients with Major Depressive Disorder (MDD).

Soterix Medical Inc. he global leader in non-invasive stimulation and synergistic technologies, announced today it has received a 510(k) clearance from U.S. Food & Drug Administration (FDA) for the Neural Navigator system manufactured by Brain Science Tools, Netherlands.

Soterix Medical Inc. announced the launch of its U.S. Food and Drug Administration (FDA) 510k cleared electro-detox™ treatment to reduce opiate and opioid withdrawal symptoms. The electro-detox™ is a battery-powered wearable device placed behind the patient’s ear that emits gentle current pulses to stimulate branches of specific cranial nerves

Soterix Medical Inc. has been awarded a Phase I NIH-SBIR contract from the National Institute of Drug Abuse (NIDA) of the National Institutes of Health (NIH) for the development of its portable remote-tDCS platform for the treatment of cocaine addiction.
The focus of the project is to develop and validate the first self-administered electrostimulation system to reduce cocaine craving. The stimulation device and protocols developed in this project are further applicable to other addictive substances like nicotine, alcohol, and marijuana and to opioid misuse.

Soterix Medical Inc. reported positive results from two double-blind, sham-controlled clinical trials of its proprietary transcranial Direct Current Stimulation-Limited Total Energy (tDCS-LTE) technology for the treatment of depression. The Depression tDCS-LTE system is unique in providing drug-free therapy with minimal side effects while maximizing energy delivery to the dorsolateral prefrontal cortex (DLPFC) region, which is implicated in depression control. The results of the first study “Bipolar Depression Electrical Treatment Trial [BETTER]” were published in JAMA Psychiatry and the results of the second study “ELECT-tDCS” trial were published in New England Journal of Medicine.

Soterix Medical Inc. announced that it has received Singapore’s Health Sciences Authority (HSA) approval for its non-invasive therapeutic medical device. This approval allows Soterix Medical to immediately market its products based on its proprietary transcranial Direct Current Stimulation (tDCS) technology in Singapore. The therapy works by delivering a mild electrical current through electrodes placed on the head relieving the symptom of Major Depression using the Depression tDCS-LTE™ treatment or Fibromyalgia using the PainX® treatment.

Soterix Medical Inc. announced it has received a 510(k) clearance from U.S. Food & Drug Administration (FDA) for its IontoDC™ device intended to use a direct current to introduce ions of soluble salts or other drugs into the body.

When the Smithsonian highlighted the most rigorous science around tDCS, they showed Soterix Medical’s exclusive High-Definition tDCS system. Targeted stimulation supporting hypothesis driven research.
Al Jazeera America features clinical grade tDCS using industry-standard Soterix Medical technology in adult and pediatric neurorehabilitation*. And an interview with our CTO, Dr. Abhishek Datta.

The Soterix Medical PainX® tDCS system receives CE Mark for the treatment of Pain including Migraine and Fibromyalgia. Soterix Medical tDCS systems continue to set the standard in research and treatment.

The Soterix Medical 1x1 tDCS system receives CE Mark for the treatment of Depression. Medical and research quality tDCS from the trusted leader in transcranial electrical neuromodulation.

Soterix Medical tDCS features on PBS inducing the industry standard 1x1 (start of episode) and our 1x1-LTE, the only device designed for susceptible population (end of episode)

Motherboard’s lighthearted take on Soterix Medical's exclusive HD-tDCS technology. Learn more about Soterix Medical HD-tDCS for state of the art research and clinical grade neuromodulation.

Soterix Medical EASYstrap features in Scientific American Mind article on “Your Electric Pharmacy”, the future of tDCS

Our exclusive "X configuration" overcomes limitations of conventional tDCS. Soterix HD-tDCS can "penetrate deeper into the brain in more focused areas", reports Nature News.
Soterix Medical Inc. partners with ElMindA Ltd. to develop a new paradigm for pain control. Visualizing individual pain networks to revolutionalize the targeted treatment of pain - one patient at a time.
For combining tDCS with monitoring technologies (such as EEG, PET, MEG, Eye-tracking, NIR, fMRI) - Soterix Medical is the only company with the technology and expertise to ensure successful integration.

WIRED magazine features Soterix medical tDCS and HD-tDCS products in their feature on neuromodulation including our exclusive 4x1 montage for brain targeting”.

Soterix High-Definition tDCS an "innovation" that "increases the functional resolution" of brain stimulation. Categorical increases in non-invasive targeting with simple to use technology.

Physicians at Harvard Medical School demonstrate the application of tDCS for clinical trial on patients using a Soterix 1x1 device in this Journal of Visualized Experiments video.
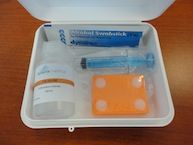
Soterix EASY-Kits include everything you need for a complete tDCS session in a compact container. Compatible with all Soterix 1x1 Stimulators and Accessories and includes Soterix EASY-Pads.
The CT-tDCS platform is the most sophisticated system for clinical trials with tDCS and the only system to provide true double-blind control and online subject monitoring.