
MEGA-TMS™
Transcranial Magnetic Stimulators
The most advanced TMS with unmatched strength and control.
Researchers and clinicians choose Soterix Medical devices and accessories where the highest standards of performance are required. Soterix Medical products stand out for their usability, unique features, and precision. Leveraging the most advanced scientific understanding, Soterix Medical technology is at the forefront of neuromodulation clinical trials for the treatment of neuropsychiatric disorders and rehabilitation.

Soterix Medical Inc. announces it has received FDA Investigational Device Exception (IDE) to launch a trial of transcranial Direct Current Stimulation-Limited Total Energy (tDCS-LTE) neuromodulation at-home for patients with Major Depressive Disorder (MDD).

Soterix Medical Inc. he global leader in non-invasive stimulation and synergistic technologies, announced today it has received a 510(k) clearance from U.S. Food & Drug Administration (FDA) for the Neural Navigator system manufactured by Brain Science Tools, Netherlands.

Soterix Medical Inc. announced the launch of its U.S. Food and Drug Administration (FDA) 510k cleared electro-detox™ treatment to reduce opiate and opioid withdrawal symptoms. The electro-detox™ is a battery-powered wearable device placed behind the patient’s ear that emits gentle current pulses to stimulate branches of specific cranial nerves

Soterix Medical Inc. has been awarded a Phase I NIH-SBIR contract from the National Institute of Drug Abuse (NIDA) of the National Institutes of Health (NIH) for the development of its portable remote-tDCS platform for the treatment of cocaine addiction.
The focus of the project is to develop and validate the first self-administered electrostimulation system to reduce cocaine craving. The stimulation device and protocols developed in this project are further applicable to other addictive substances like nicotine, alcohol, and marijuana and to opioid misuse.

Soterix Medical Inc. reported positive results from two double-blind, sham-controlled clinical trials of its proprietary transcranial Direct Current Stimulation-Limited Total Energy (tDCS-LTE) technology for the treatment of depression. The Depression tDCS-LTE system is unique in providing drug-free therapy with minimal side effects while maximizing energy delivery to the dorsolateral prefrontal cortex (DLPFC) region, which is implicated in depression control. The results of the first study “Bipolar Depression Electrical Treatment Trial [BETTER]” were published in JAMA Psychiatry and the results of the second study “ELECT-tDCS” trial were published in New England Journal of Medicine.

Soterix Medical Inc. announced that it has received Singapore’s Health Sciences Authority (HSA) approval for its non-invasive therapeutic medical device. This approval allows Soterix Medical to immediately market its products based on its proprietary transcranial Direct Current Stimulation (tDCS) technology in Singapore. The therapy works by delivering a mild electrical current through electrodes placed on the head relieving the symptom of Major Depression using the Depression tDCS-LTE™ treatment or Fibromyalgia using the PainX® treatment.

Soterix Medical Inc. announced it has received a 510(k) clearance from U.S. Food & Drug Administration (FDA) for its IontoDC™ device intended to use a direct current to introduce ions of soluble salts or other drugs into the body.

When the Smithsonian highlighted the most rigorous science around tDCS, they showed Soterix Medical’s exclusive High-Definition tDCS system. Targeted stimulation supporting hypothesis driven research.
Al Jazeera America features clinical grade tDCS using industry-standard Soterix Medical technology in adult and pediatric neurorehabilitation*. And an interview with our CTO, Dr. Abhishek Datta.

The Soterix Medical PainX® tDCS system receives CE Mark for the treatment of Pain including Migraine and Fibromyalgia. Soterix Medical tDCS systems continue to set the standard in research and treatment.

The Soterix Medical 1x1 tDCS system receives CE Mark for the treatment of Depression. Medical and research quality tDCS from the trusted leader in transcranial electrical neuromodulation.

Soterix Medical tDCS features on PBS inducing the industry standard 1x1 (start of episode) and our 1x1-LTE, the only device designed for susceptible population (end of episode)

Motherboard’s lighthearted take on Soterix Medical's exclusive HD-tDCS technology. Learn more about Soterix Medical HD-tDCS for state of the art research and clinical grade neuromodulation.

Soterix Medical EASYstrap features in Scientific American Mind article on “Your Electric Pharmacy”, the future of tDCS

Our exclusive "X configuration" overcomes limitations of conventional tDCS. Soterix HD-tDCS can "penetrate deeper into the brain in more focused areas", reports Nature News.
Soterix Medical Inc. partners with ElMindA Ltd. to develop a new paradigm for pain control. Visualizing individual pain networks to revolutionalize the targeted treatment of pain - one patient at a time.
For combining tDCS with monitoring technologies (such as EEG, PET, MEG, Eye-tracking, NIR, fMRI) - Soterix Medical is the only company with the technology and expertise to ensure successful integration.

WIRED magazine features Soterix medical tDCS and HD-tDCS products in their feature on neuromodulation including our exclusive 4x1 montage for brain targeting”.

Soterix High-Definition tDCS an "innovation" that "increases the functional resolution" of brain stimulation. Categorical increases in non-invasive targeting with simple to use technology.

Physicians at Harvard Medical School demonstrate the application of tDCS for clinical trial on patients using a Soterix 1x1 device in this Journal of Visualized Experiments video.
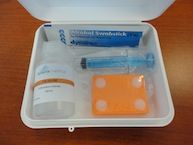
Soterix EASY-Kits include everything you need for a complete tDCS session in a compact container. Compatible with all Soterix 1x1 Stimulators and Accessories and includes Soterix EASY-Pads.
The CT-tDCS platform is the most sophisticated system for clinical trials with tDCS and the only system to provide true double-blind control and online subject monitoring.
The study team is investigating whether or not transcutaneous auricular vagus nerve stimulation (taVNS) can treat neurologic symptoms of COVID-19 which are termed NEUROCOVID.
The purpose of this study is to assess the feasibility, acceptability, and efficacy of self-administered transcranial direct current stimulation (tDCS) in older adults with knee osteoarthritis (OA)
This study will evaluate the feasibility and efficacy of home-administered transcranial Direct Current Stimulation (tDCS) treatment for depression.
FDA IDE trial of transcranial Direct Current Stimulation-Limited Total Energy (tDCS-LTE) neuromodulation at-home for patients with Major Depressive Disorder (MDD).
This study will investigate the effects of transcutaneous direct current stimulation (tsDCS) on walking function in individuals with incomplete spinal cord injury.
Pragmatic clinical trial that aims to determine the effect of tDCS on symptomatic fatigue in Multiple Sclerosis (MS) patients. This is a randomized, blinded, sham-controlled study design to determine the effect of Transcranial Direct Current Stimulation (tDCS) on MS participants to reduce feelings of fatigue.
Due to the limited effectiveness of conventional speech therapy strategies in post-stroke aphasia an effective novel treatment is therefore needed to improve recovery in these patients. A double-blind randomized controlled design will be conducted. Participants will randomly be assigned either to the tDCS group or to the sham (placebo) group.
Study aims to understand and determine how tDCS works, how it can be optimized, and if it can be used as an effective intervention for reducing depressive symptoms in people suffering from Major Depression
Trial will assess the individual and combined impact of pairing cognitive training with tDCS in adults (65-89 years).
Soterix Medical 1x1 Clinical Trials (CT) device using Limited Total Energy (LTE™) technology is being used in the study.
This study evaluates the application of non-invasive brain stimulation in the treatment of Mild Cognitive Impairment (MCI) in adults aged 55-85.
Soterix Medical 1x1 tES device is being used in the study.
This study explores the effects transcranial Direct Current Stimulation on Phantom Limb Pain for patients experiencing chronic phantom limb pain in open-label study design.
Soterix Medical 1x1 tDCS device is being used in the study.
The purpose of the pilot study will be to evaluate the feasibility of open label Transcranial direct current stimulation (tDCS) in combination with computerized cognitive behavior therapy (cCBT) to maintain wellness following an acute course of Electroconvulsive therapy (ECT) for up to 6 months.
Soterix Medical 1x1 tDCS mini-CT device is being used in the study
Soterix Medical 1x1 Clinical Trials device with Limited Total Energy (LTE) to be used in a trial to study the antidepressant effects of tDCS in bipolar disorder.
Hunter College of The City University of New York will investigate the use of Soterix Medical 1x1 tDCS (transcranial direct current stimulation) in conjunction with speech-language therapy, for the improvement of language production in stroke survivors with aphasia.
Trial will compare Soterix Medical tDCS-LTE™ technology against a fully dosed, effective antidepressant Escitalopram oxalate (Lexapro). Recruitment ongoing.
Soterix Medical 1×1 Clinical Trials (CT) device using Limited Total Energy (LTE™) technology is being used in the study.
Trial will assess the clinical and neurophysiological effects of a non-invasive brain stimulation technique - transcranial direct current stimulation (tDCS)- on cortical plasticity and motor learning in children with cerebral palsy.
This study will evaluate the influence of non-invasive brain stimulation on different elements of cognitive function in healthy persons between the ages of 18-90 years. Soterix Medical 1×1 tDCS device is being used in this study.
Spaulding Rehabilitation Hospital and the US Department of Education will investigate the effects of Soterix Medical 1x1 transcranial direct current stimulation (tDCS) on the pain and itching associated with burn injury. This study is part of the Boston-Harvard Burn Model System.
Follow up Phase II, HD-tDCS clinical trial underway for fibromyalgia at Harvard Medical School and Spaulding Rehabilitation Hospital, and in conjunction with Elminda Corporation. Exclusive Soterix 4x1 HD-tDCS being used.
This study is testing whether the addition Soterix Medical 1x1 transcranial direct current stimulation (tDCS) when combined with meditation helps decrease the abdominal pain in patients with chronic pancreatitis.
Study focused on investigating effect of tDCS on chronic corneal pain as well as changes in thought processing as compared to healthy controls. Soterix 1×1 Device is being used.
Study focused on evaluating the effect of a combination of robotic arm therapy and transcranial Direct Current Stimulation for greater functional recovery after stroke.
For the most comprehensive tDCS trial to-date, researchers selected the most advanced tDCS device and the only system optimized for clinical trials – The Soterix Medical 1x1-CT.
Epilepsy Foundation awards a New Therapy Grant for a clinical trial of High-Definition tDCS in pediatric epilepsy. Dr. Alexander Rotenberg will lead a team of clinical investigators including at BCH and Harvard Medical School.
NIH grants Burke Medical Research Institute $3m to conduct the most controlled trial of tDCS for stroke rehabilitation to date. The Soterix Medical 1x1 platform is the most advanced and the industry standard for tDCS clinical trials for rehabilitation.
Soterix Medical Inc. receives FDA Clinical Trial IDE for High-Definition tDCS (HD-tDCS) to improve IQ in pediatric Down syndrome. HD-tDCS is the only non-invasive, brain targeted, and low-intensity technology designed to promote neuroplasticity.
Dr. Bernadette Gillick's Pediatric Rehabilitation Laboratory is supported by the NIH and University of Minnesota Clinical and Translational Science Institute to investigate the use of a form of tDCS for interventions in rehabilitation for children. Subject specific analysis with Soterix Medical Neurotargeting™ will guide current to brain cells in the injured part of the brain.
HD-tDCS clinical trial underway for fibromyalgia at Harvard Medical School and Spaulding Rehabilitation Hospital, Boston.
NIH grants Soterix a Phase-1 STTR grant to develop HD-tDCS for stroke rehabilitation. The first technology capable of delivering therapeutic direct current to target brain regions involved in functional recovery.