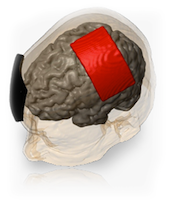
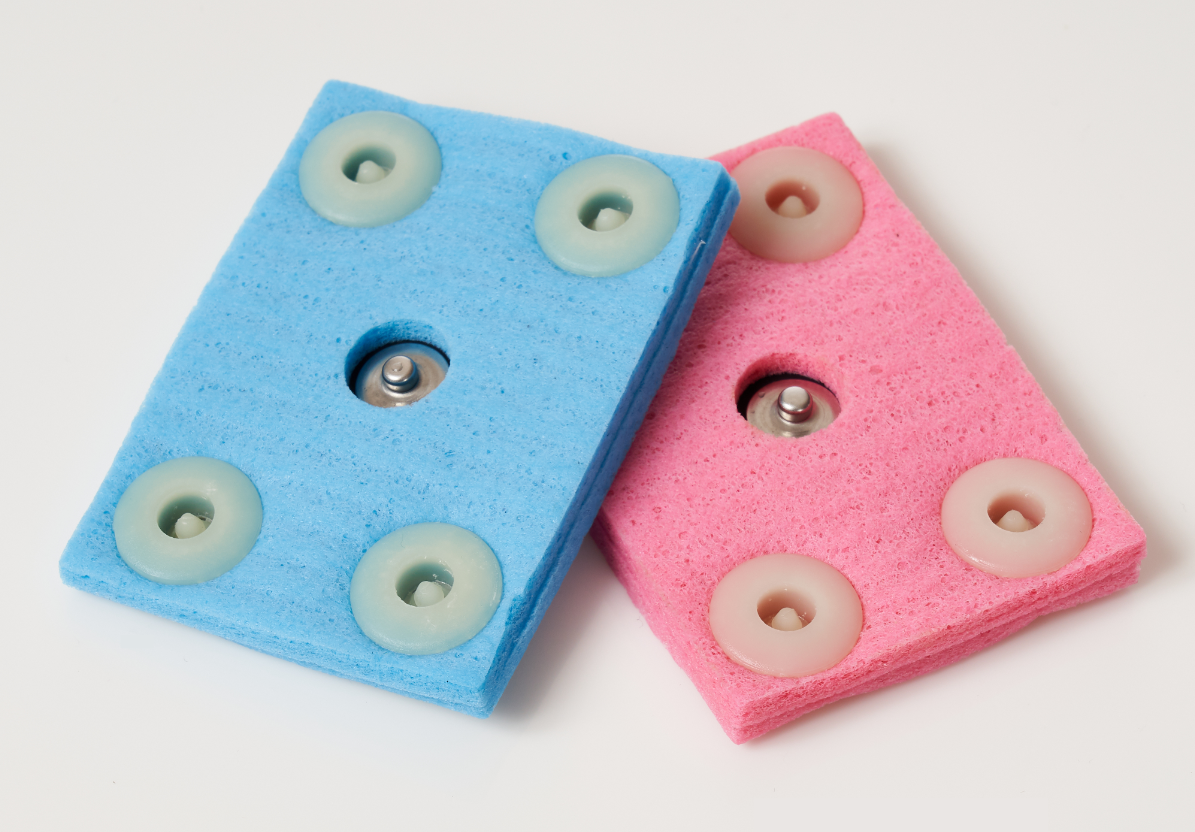
Transcranial Direct Current Stimulation (tDCS) is a non-invasive procedure in which a device sends a small Direct Current (DC) across the scalp to modulate brain function. The Soterix Medical 1x1 tDCS Low-Intensity Stimulator sends a low-level current from the positive electrode, anode, to the negative electrode, cathode. When the extremely low level current passes from the anode to the cathode, it may simultaneously increase the activity of the brain by the anode and decrease the activity of the brain near the cathode.
tDCS mechanisms are considered to result from the ability of very weak DC currents to safely induce reversible changes in cortical plasticity. The induction of lasting changes in cortical excitability can, under some conditions, reversibly modify behavior and interact with normal learning. Such findings have driven a large number of studies examining whether tDCS might induce functionally significant changes in patients with a large variety of neurological and psychiatric disorders.
tDCS dose can be defined as: 1) The size and position of the electrodes on the body and 2) The duration (in minutes) and intensity (in mA) of current passed across the electrodes. Soterix Medical tDCS systems allow precise reproduction of tDCS doses commonly used in medical literature. Soterix Medical engineers can work with you to determine the best configuration for your application. tDCS is an investigational technique and it is the responsibility of the operator to determine the appropriate tDCS dose.
tDCS safety is supported by medical literature to have common side effects limited to mild and reversible skin irritation when using standard tDCS protocols and guidelines. Soterix Medical tDCS stimulators and electrodes are uniquely designed to minimize skin irritation – for example, the exclusive SMARTscan feature provides a simple indicator to the operator of the contact conditions before, during, and after stimulation. tDCS is an investigational technique, and it is the responsibility of the operator to identify and follow the most appropriate safety protocols.
tDCS comfort can be controlled by the operator by using devices, such as the Soterix Medical 1x1 tDCS Low-Intensity Stimulator, which are specifically designed for clinical tDCS. For example, the unique PRE-STIM TICKLE and RELAX features available on all Soterix Medical 1x1 models are designed to condition the skin prior to stimulation and allow the operator to accommodate subject feedback without stopping stimulation.